The future of single-use, column-free purification is here.
Revolutionizing bioprocessing productivity, product quality, and recovery with continuous chromatography.
Scalable Intensification
Enabling intensification and continuous purification of the most challenging biological modalities
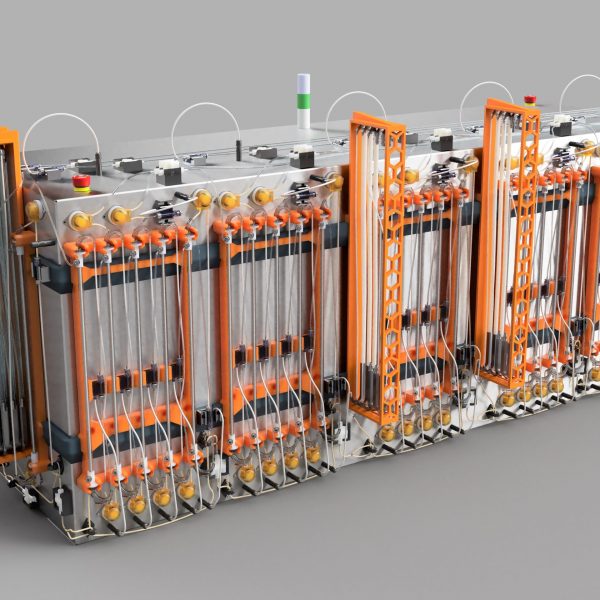
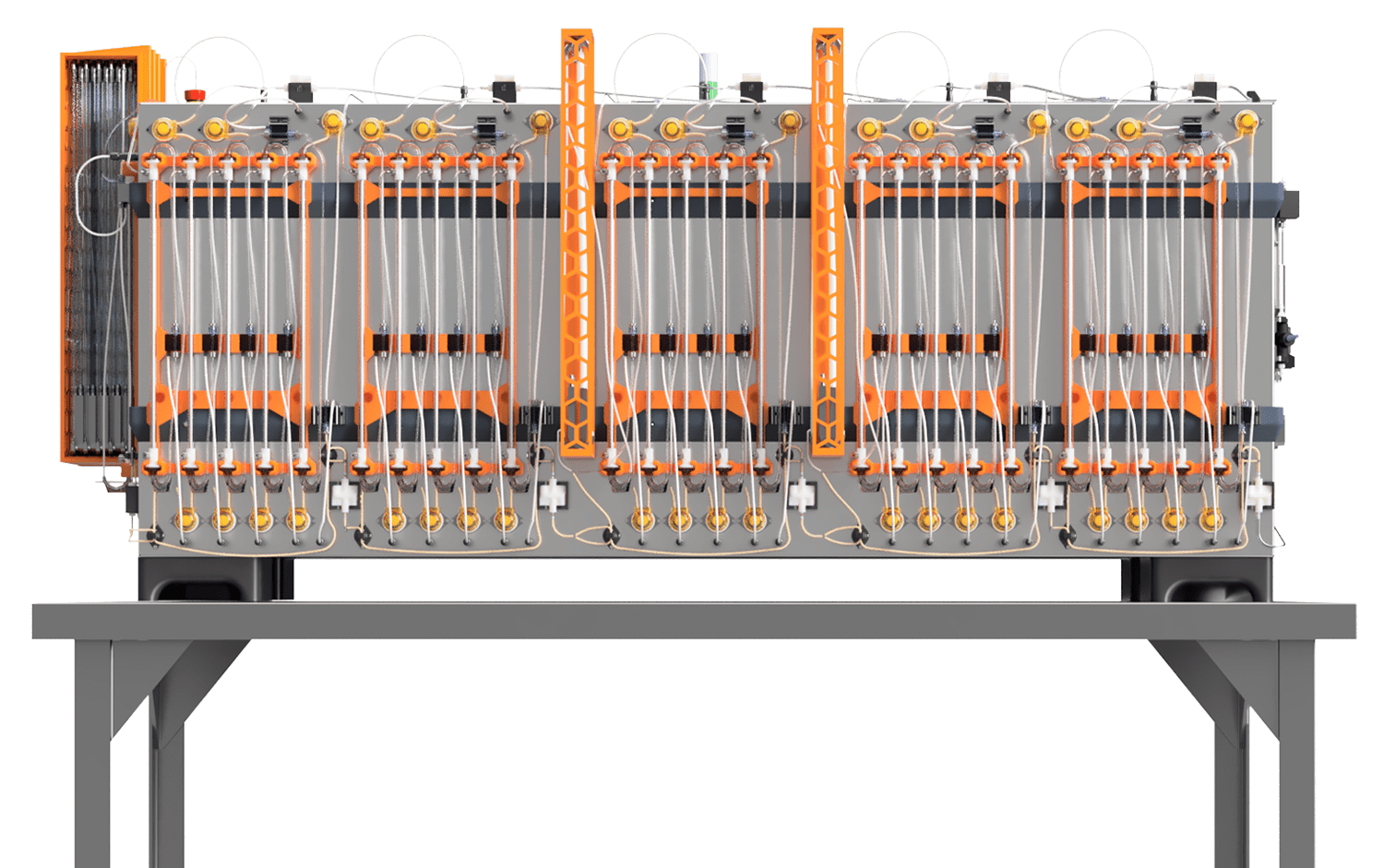
Meet the revolutionary Kascade™ BioRMB™— a column-free chromatography platform engineered for the future of biomanufacturing. This groundbreaking system features true steady-state, low-pressure operation, 10X resin volume reduction, and up to 70% OpEx savings. With an easy-to-install, disposable, and fully closed flowpath, BioRMB™ eliminates the need for packing, cleaning, and resin storage, significantly simplifying validation requirements, and eliminating lengthy installation and qualification procedures.
Utilizing true moving bed technology and multi-stage countercurrent extraction, BioRMB™ dramatically enhances process efficiency, enabling rapid resin cycling of 3-5 cycles per hour. In addition, the platform can use small particle-size resins for improved kinetics and higher binding capacity. Especially advantageous for sensitive modalities—including gene therapy products, fusion proteins, DNA, and mRNA—BioRMB™ ensures higher recoveries, superior product quality, minimal resin contact time, low pressure and shear, as well as in-line dilution and instantaneous elution chemistry adjustments.
With its reduced footprint and hyper-efficiency, BioRMB™ paves the way for facilities of the future.
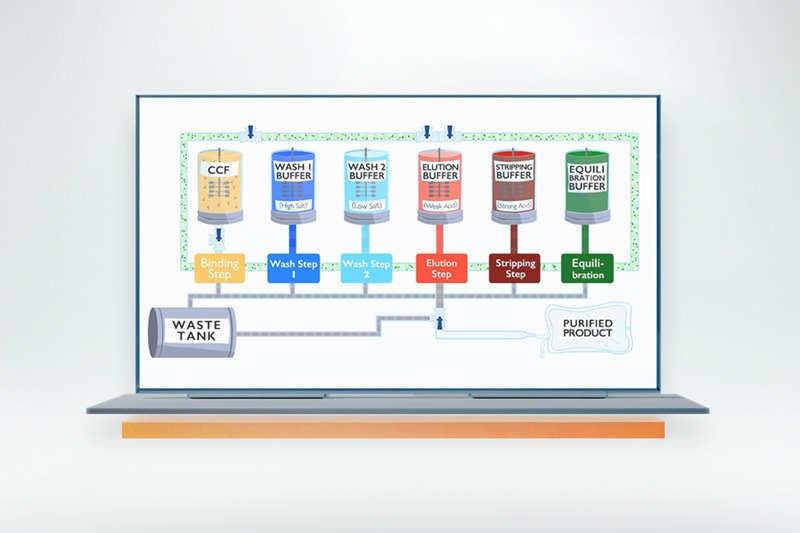
True moving bed Technology enabling superior efficiency and dramatically reduced resin consumption.
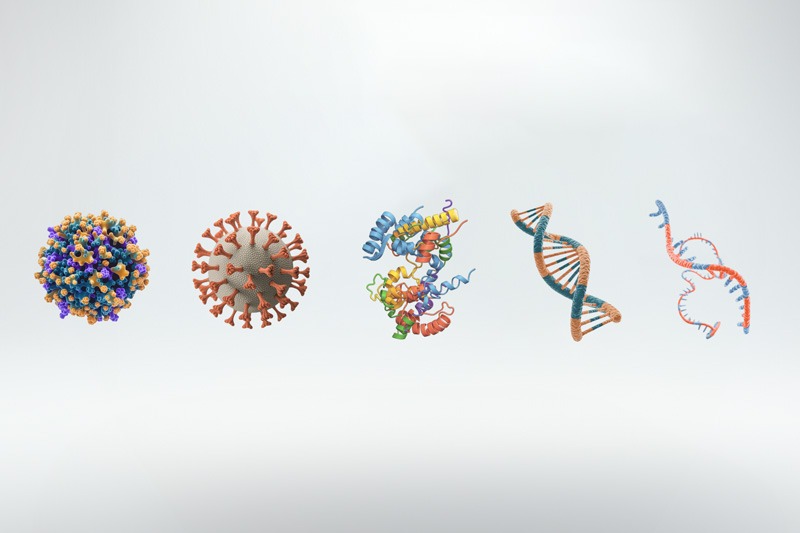
Explore Applications for sensitive modalities such as gene therapy products, fusion proteins, DNA, and mRNA.